Serving ten rural Kentucky counties, Baptist Health Hardin adds 3D Ion® bronchoscopy for lung cancer screening, and is the first to begin in-human trials of new 4D mammography technology
ELIZABETHTOWN Advancements in radiology and imaging are happening in a big way at Baptist Health Hardin in Elizabethtown, Kentucky.
Baptist Health Hardin is the first hospital to begin in-human clinical trials of a revolutionary breast imaging technology that could transform the way breast cancer is detected and diagnosed.
This first clinical trial is expected to enroll approximately 60 patients over the next several months. The study is being led by principal investigator Craig Kamen, MD, chair of the imaging service line for Baptist Health System. Kamen is also president of Radiology Associates, Inc., a 50-person-plus radiology practice.
“As primary investigator, I ensure regulatory compliance and appropriate candidates for the study with the help of the most amazing research team at Baptist Health. This is one of the first radiology research studies we’re doing within the system,” says Kamen.
Kamen received his medical degree at the Medical College of Ohio, in Toledo. He did his diagnostic radiology residency at Mount Sinai Medical Center, in Cleveland and then a two-year neuro-radiology fellowship at University Hospitals/Case Western Reserve.
The Need for Advanced 4D Imaging
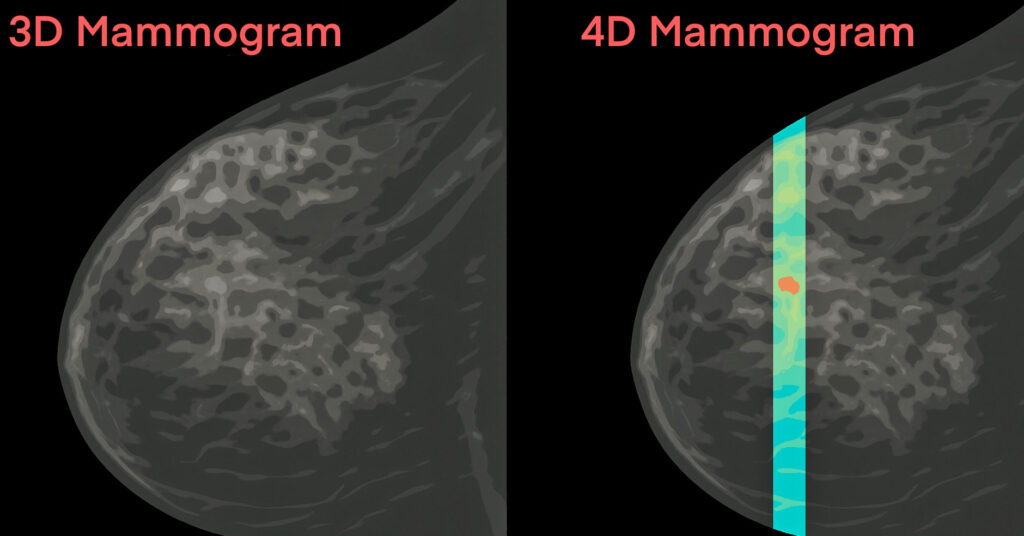
Calidar, Inc., a pioneer in precision diagnostic imaging, developed the world’s first clinical system for X-ray diffraction imaging of breast cancer. The investigational 4D mammography device reads molecular level “fingerprints” in tissue, offering information that is not available using currently approved imaging technology.

Breast cancer remains difficult to diagnose without invasive procedures, leading to millions of unnecessary biopsies and delayed care each year. Using 4D mammography, researchers will test the technology’s potential ability to identify cancers earlier and more accurately with the goal of giving doctors and patients better answers, faster.
The trial launched in August 2025 with the imaging of the first research volunteer, using the 4D mammography system designed by Stefan Stryker, Josh Carpenter, and Mitchell Greene of Calidar, Inc. The device uses X-ray diffraction to measure how X-rays scatter at the molecular level, producing a unique “signature” that reflects the internal composition of breast tissue. Unlike traditional X-ray images which rely on shape and density, this technology reveals what the tissue is made of — not just what it looks like — offering a new dimension of diagnostic data.
Kamen states, “There are times we see findings on mammograms that we’re not sure if it’s potentially malignant or benign. That’s when we decide to do a breast biopsy. The hope would be that by using X-ray diffraction, we may be able to discriminate between benign and malignant tissue and do fewer biopsies or limit follow-up diagnostic imaging exams.”
“This is more than a study milestone — this is the start of a new era of medical imaging,” says Stryker, CEO of Calidar. “X-ray diffraction has unlocked some of the most iconic achievements in science — from discovering the structure of DNA to revealing the composition of another world on the Mars rover — and now we are bringing its power into the clinic to look inside the human body in a completely new way. Our 4D mammography system brings this capability to the challenge of breast cancer diagnostics where high-precision noninvasive imaging tools are urgently needed.”
Stryker is from Wilmore, Kentucky. When his North Carolina-based company developed the novel X-ray diffraction technology for breast cancer detection, he reached out to local connections that he had with some of the administration at Baptist Health Hardin and brought both parties together. The hospital has a large presence in women’s imaging in Central Kentucky, and they had the space and the personnel to carry out the study.
“We are honored to be participating in this first-in-human clinical trial focusing on advancing care for mammography patients,” said Bert Jones, the hospital’s director of medical imaging. “This groundbreaking research has the potential to change the course of treatment for countless people. Being part of this pivotal moment in medical innovation represents more than just scientific process — it’s a step forward in improving the lives of women everywhere.”
“Baptist Health Hardin is committed to ensuring our patients have access to next generation technology,” says hospital president Robert Ramey. “Being chosen as the first hospital to conduct in-human trials of 4D mammography reflects the dedication of our physicians and staff to setting a new standard in patient care.”
Study Criteria Parameters and Benefits
Study volunteers will meet specific criteria following 3D screening and diagnostic imaging that would typically lead to a biopsy. Volunteers must be at least 22 years of age, between 4’10” and 6’2″ inches in height; be able to stand for five minutes; and have had a digital breast tomosynthesis diagnostic exam in the last 90 days with an abnormality of at least three millimeters or larger in size and eight millimeters from the chest wall.
Data from the trial will help determine the system’s effectiveness and guide future studies including potential use in routine breast cancer screening. Data gathered will not impact or influence the routine diagnosis and treatment of the study volunteers.
Kamen points out multiple benefits that could come from 4D mammography research. “We will save time, money, and patients’ anxiety. That’s where the promise lies within this technology; that we may be able to mitigate those patients that fall in the middle of ‘not sure it’s malignant, and not sure it’s benign,’” he says.
Baptist Health Foundation Hardin supported the project by funding a portion of the necessary renovations to house the new 4D mammography equipment and services.
The 4D mammography system is investigational and has not been cleared or approved by the U.S. Food and Drug Administration. It is not available for commercial sale and is limited to investigational use in the United States under FDA’s abbreviated IDE requirements.
Baptist Health Hardin Now Offering 3D Robotic-Assisted Bronchoscopy
Baptist Health Hardin has launched a powerful new upgrade to its Ion® bronchoscopy program: 3D imaging technology.
The upgrade, considered the gold standard in lung cancer diagnosis, makes the Elizabethtown hospital one of just a few programs in the state to offer this level of diagnostic precision in lung cancer care.
With Kentucky leading the nation in lung cancer incidence and smoking rates, this investment offers transformative potential. The upgraded 3D Ion system enables physicians to identify and biopsy small lung nodules—often located in hardto-reach outer areas of the lung with greater speed, precision, and safety.
“Lung cancer remains the deadliest cancer in America, and the reality is that too many patients are diagnosed at later stages,” says Ramey, hospital president. “This technology helps us change that story—right here, close to home.”
Finding lesions early, when they are smaller, allows for the most treatment options and improved patient outcomes.
“This technology transforms how we find and treat lung nodules,” says Aaron Mulhall, MD, a physician with Baptist Health Medical Group Pulmonary & Critical Care. “It’s like finding a needle in a haystack—but the haystack is always moving. The enhanced 3D capability allows us to lock in on the nodule quickly and accurately, giving patients the best chance at a full, thriving life after cancer.”
The hospital has already performed more than 700 lung screening procedures since Ion was introduced in 2022 and anticipates continued growth in the number of patients served through this upgraded technology.
These advancements reflect Baptist Health Hardin’s mission to deliver excellence in care. In smaller, rural regions where early detection
can be the difference between a full, healthier life and serious health complications, these investments are deeply personal.
“Our goal is to give every person in our community the greatest possible chance—not just to survive cancer, but to truly thrive beyond it,” says Ramey. “When we invest in advanced care, we’re not only supporting individuals on their cancer journey—we’re strengthening families, uplifting neighborhoods, and improving the health of our entire region.”